Researcher Guide for Projects
Project Initiation Process
- The Principal Researcher is responsible for ensuring that projects under the HECW program align with the HECW aims, and adhere to the program processes, including ensuring that every member of the research team understands, and is accountable for the ethical conduct of research, and the protection of the collection, use and disclosure of data across the research lifecycle, according to their role, TMU policies, defined HECW procedures outlined this DMP, jurisdictional legislation, and Tri-Council Agency policies, practices and procedures, across the project and data management lifecycle.
- The TMU Research Ethics Board will be responsible for the approval of all projects relating to the collection, use and disclosure of data relating to the protection of human subjects’ rights.
- Principles of equity, diversity, inclusivity and accessibility (EDIA), community engagement and knowledge sharing are integrated into the HECW program. These principles will be integrated across the project and data management lifecycle, especially for projects involving Canadian Indigenous communities.
- Research projects are approved, and associated data are collected, processed, analyzed, archived, stored and re-used, based on pre-approved project plans and approvals, research participant consent, and according to best practice research conduct throughout the research and data management lifecycle.
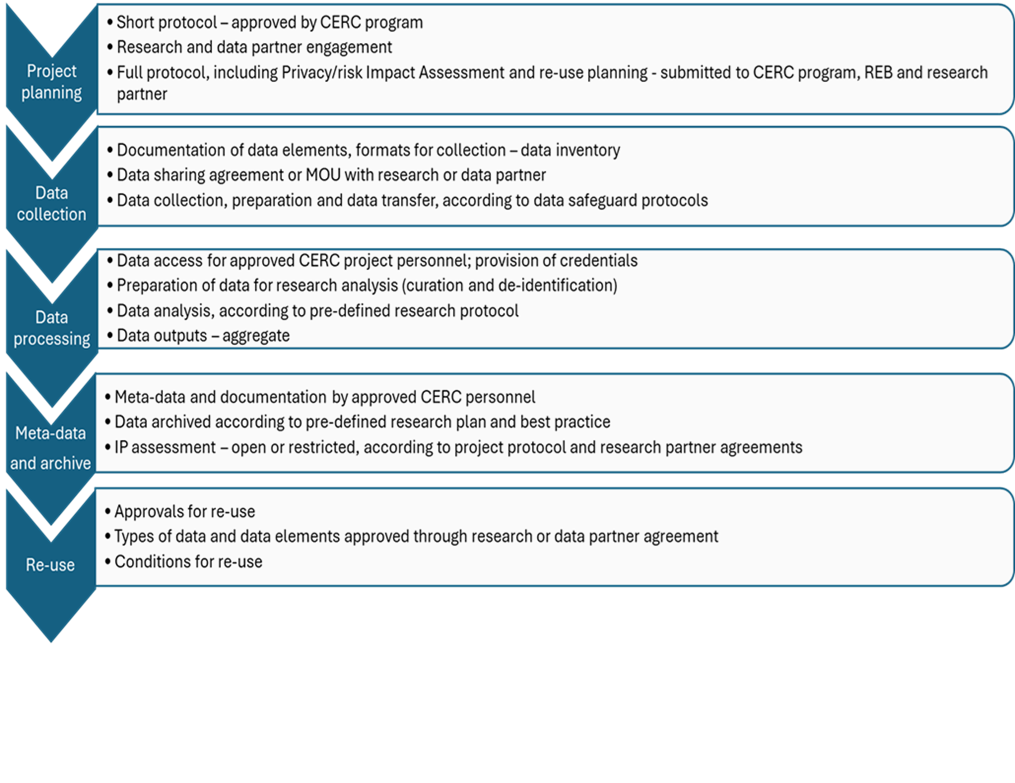
- The broad steps to be taken are:
- Project planning, including partner engagement and staff training
- REB approval
- Data Collection
- Data processing, meta-data and archiving
- Data re-use and project close
- Community engagement: Research partners, whether academic, external organizations, or communities, will have been already engaged, with their input into the project ideas, design, community engagement plan, data management plan, and knowledge mobilization plans discussed and agreed upon.
- Short Protocol: Please complete an (google form) initial short project protocol (external link, opens in new window) that will automatically be submitted to Prof. Soldatić for her and her operational team’s review. This step should take no more than 15 minutes. This step is to ensure that your project is aligned with the CERC-HECW mandate, to assist the CERC-HECW in providing you with the initial resources and support you may need to initiate your project, and to confidentially collect project information for operational tracking and auditing purposes. Only Prof. Soldatić and the CERC Program Operations and Data Manager will have access to this information.
- Approval in principle – next steps: You will hear back from the CERC-HECW office within 10 days of your short form submission regarding next steps for your project. You will receive a project number that will be used for communication with the CERC-HECW until project closure.
- Training: Principal Investigators and project leads are responsible for the required training of all research staff and teams.
- A list of training/learning modules is provided for CERC-HECW researchers and students. These modules should be reviewed, and their concepts understood by all persons who are engaged within your research project.
- If your project includes community engagement, please ensure that *the community engagement learning module (external link, opens in new window) is completed.
- If your project involves Indigenous Peoples of Canada, or their data, respect for research self-governance and data sovereignty is required according to the Tri-Council Policy Statement, Chapter 9 (external link, opens in new window) .
- Full project protocol: After initial approval by the CERC Health Equity and Community Wellbeing, a detailed project protocol will be expected.
- The full project protocol must include:
- Community engagement plan
- EDIA plan
- Knowledge mobilization plan
- Data Management and Privacy Impact plan
- Plan if involving Indigenous communities or data
- Project close out plan
- The full project protocol must include:
- Research Ethics Board Submission: The process for research ethics board submission is described below.
- Data collection and use will be approved through appropriate research participant consent, a data sharing agreement from an external organization, and/or appropriate legislative authority.
- Data transfer/collection will only be considered through a demonstrated and secure means.
- No identifiable sensitive or personal information will be collected or used for any project.
- Each project will complete a Data Management Plan (DMP), including Privacy Impact Plan. Its completion will ensure that research project team and members understand and has documented the applicable data management requirements, and that no relevant aspect of data management is overlooked.
The CERC-HECW DMP is an online google form. The information captured is stored with the CERC Operations and Data Manager and will be used for data governance and auditing purposes. The DMP/PIA includes information such as:
o Rational for collecting project data, relating to project objectives
o Types of data sources (such as: clinical inputs from health care providers, interview, focus group and survey data from respondents, routinely collected administrative from data providers, community-level data (local or community program information), social determinants indicator data, media such as photographs, historical notes, and art outputs)
o Characteristics of data and data elements (aggregate, individual level, health, geographic variables, demographic etc)
o Names of data custodians and data transfer protocol, if data are secondary use
o Community engagement – who has ownership and stewardship of data
o Authority for data use (consent, legal and legislative)
o Data collection method
o Data platform for data analysis and storage
o Metadata and rules for re-use
o Types of software/hardware needed
o Names of those accessing data and their roles and responsibilities
o Initiation and termination dates for accessing data
o Data access initiation and termination dates
o Plans for data retention and destruction
o REB process, based on type of data
o Method and type of information disclosed for knowledge sharing
o Requirements by academic journals
- Data collection, analytic space will be required to be on a secure data platform; the platform managed by the Digital Research Alliance of Canada (the Alliance) could be used for de-identified data; analytic software is also available on this system. Allocated space for the HECW data has been approved by the Alliance and will be managed according their technical specifications (external link, opens in new window) , and policies, practices and procedures (external link, opens in new window) . Data access will be approved by the CERC and will follow the Alliance defined processes and controls.
- Data use considerations:
- Sensitive or personal data will be collected only as required for the project. Secure and private controls for these data will be described in the research protocol, collected through consent, and approved by the CERC and the TMU and/or institutional REB.
- Meta data will be catalogued according to formats provided by Borealis, supported by TMU (TMU Dataverse (external link, opens in new window) and R-share (opens in new window) , for example).
- Re-use of data will be upon approval and research participant consent only, and according to the defined HECW program.
- All researchers will have obtained the necessary credentials to fulfill their responsibility relating the project needs and will have reviewed the provided training materials focusing on data stewardship.
- Sensitive data, defined as participant-level clinical or first-hand accounts, or data involving Indigenous Peoples, will be collected through consent only, and through full REB approval.
- Appropriate de-identification protocols will be employed so that individual or community identification, with or without additional data, is not reasonably possible – privacy protecting protocol training will be available from the Alliance.
- The collection of individual-level data will be through a random research project identification number (PIN); no community-level or personally identifiable data will be collected or processed without pre-approved rationalization to do so.
- If a crosswalk of personally identifiable data and PINs is needed, according to the study protocol, and approved by the REB, no clinical or personal data will be collected on the crosswalk data file. Only one identified person will have access to this crosswalk. The storage of the crosswalk and de-identifiable data for analysis will have no ability for cross-reference, either through hardware or access limits.
- Where appropriate to the project and data collection purpose, research protocol and consent, separation of data collection and data analysis duties will be employed so that those analyzing the data are unable to identify participants.
- The source and nature of potential risks must be documented with an assessment of likelihood and severity of harm, and assessment of overall risk. A privacy impact assessment that is integrated into the DMP has been developed in collaboration with TMU Privacy Office.
- Only non-identifiable and aggregated data will be outputted and used for knowledge sharing and publication. The CERC and research leads will review all knowledge outputs to ensure anonymity and research integrity.